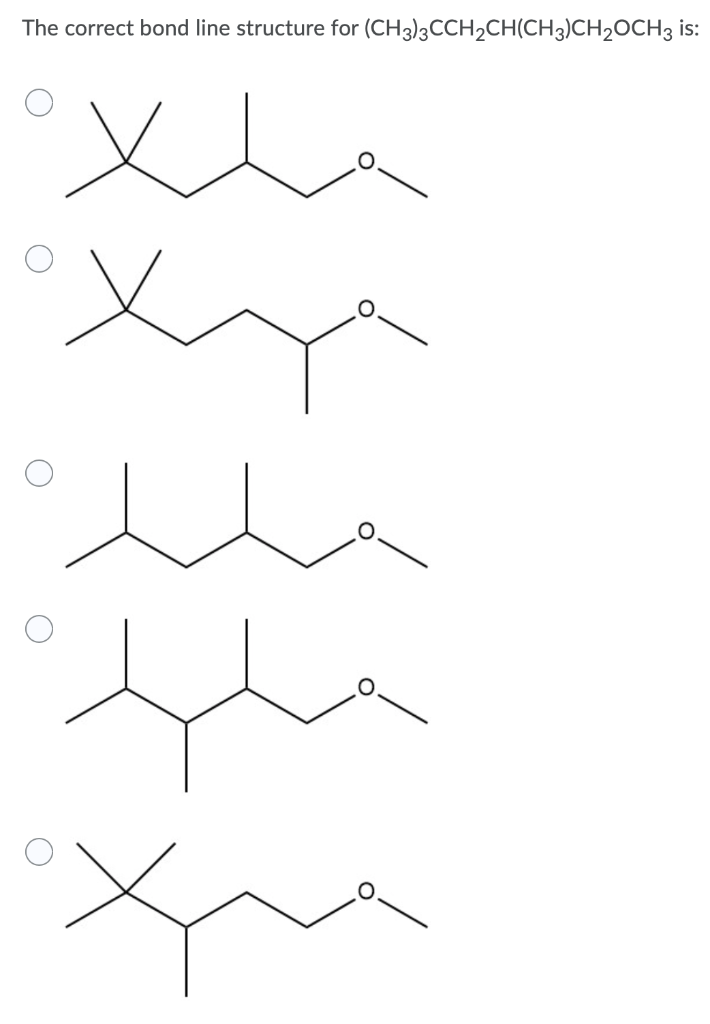
Drawing the Line Structure of CH3CH2COOCH2CH(CH3)2
In the realm of organic chemistry, understanding the intricate architecture of molecules is paramount. These structures dictate their properties and reactivity, shaping the world around us. One such molecule, CH3CH2COOCH2CH(CH3)2, commonly known as ethyl 3-methylbutanoate, is of particular interest. Let us embark on a journey to elucidate its line structure, unlocking the secrets hidden within its molecular framework.
Deciphering the Structural Formula
The line structure of CH3CH2COOCH2CH(CH3)2 can be represented as follows:
CH3-CH2-CO-O-CH2-CH(CH3)-CH3
This formula reveals the arrangement of atoms within the molecule, depicting the carbon-carbon bonds, hydrogen-carbon bonds, and various functional groups present. It serves as a roadmap, guiding us through the molecular landscape.
The molecule comprises a central carbonyl group (C=O), which is characteristic of esters. This group consists of a carbon atom double-bonded to an oxygen atom. The carbon atom of the carbonyl group is further bonded to an ethyl group (CH3CH2) and an oxygen atom of the ester group (COOCH2CH(CH3)2). The ester group, in turn, is attached to a 3-methylbutyl group (CH2CH(CH3)2).
Navigating the Molecular Landscape
The line structure provides insights into the molecular architecture of CH3CH2COOCH2CH(CH3)2. It reveals the presence of several structural features, each contributing to the molecule’s overall properties and reactivity.
The carbonyl group, with its polar double bond, is a key functional group responsible for the molecule’s reactivity. It can undergo nucleophilic attack, making it susceptible to reactions with a variety of reagents. The ester group, on the other hand, provides the molecule with its characteristic fruity aroma and contributes to its solubility in organic solvents.
Latest Trends and Developments
In recent years, there has been growing interest in the use of CH3CH2COOCH2CH(CH3)2 as a biofuel. Its high energy content and relatively low volatility make it a promising alternative to fossil fuels. Researchers are exploring ways to optimize its production and utilization, paving the way for sustainable energy solutions.
Additionally, advancements in analytical techniques have enabled researchers to probe the molecular structure of CH3CH2COOCH2CH(CH3)2 with unprecedented detail. These techniques provide insights into its conformational changes, intermolecular interactions, and dynamic behavior, shedding light on its complex molecular dynamics.
Tips and Expert Advice
For those seeking to delve deeper into the fascinating world of CH3CH2COOCH2CH(CH3)2, here are some invaluable tips:
- Consult reputable scientific databases and textbooks for comprehensive information on the molecule’s structure, properties, and reactivity.
- Attend scientific conferences and workshops to engage with experts in the field and stay abreast of the latest advancements.
- Use molecular modeling software to visualize the 3D structure of CH3CH2COOCH2CH(CH3)2 and explore its conformational flexibility.
- Stay informed about the ongoing research and applications of CH3CH2COOCH2CH(CH3)2 in various scientific disciplines.
By following these tips, you can enhance your understanding of this molecule and its significance in the scientific community.
Frequently Asked Questions (FAQs)
To further clarify your understanding of CH3CH2COOCH2CH(CH3)2, here are some commonly asked questions:
- Q: What is the molecular weight of CH3CH2COOCH2CH(CH3)2?
A: The molecular weight of CH3CH2COOCH2CH(CH3)2 is approximately 130 g/mol. - Q: Is CH3CH2COOCH2CH(CH3)2 soluble in water?
A: CH3CH2COOCH2CH(CH3)2 is sparingly soluble in water due to its hydrophobic nature. - Q: What is the boiling point of CH3CH2COOCH2CH(CH3)2?
A: The boiling point of CH3CH2COOCH2CH(CH3)2 is approximately 165°C. - Q: What is the IUPAC name of CH3CH2COOCH2CH(CH3)2?
A: The IUPAC name of CH3CH2COOCH2CH(CH3)2 is ethyl 3-methylbutanoate.
Conclusion
The line structure of CH3CH2COOCH2CH(CH3)2 provides a blueprint for understanding its molecular architecture and properties. By deciphering its structural formula, we gain insights into the arrangement of atoms, functional groups, and their interconnections. Through ongoing research and advancements in analytical techniques, we continue to unravel the complexities of this molecule, paving the way for novel applications and scientific breakthroughs.
If you found this article informative, please share your thoughts and questions in the comments section below. Your feedback helps us refine our content and provide the best possible learning experience for our readers.
Image: chegg.com

Image: homework.study.com
Answered: 2. Draw complete Lewis structure and… | bartleby structure diagram of CH3 (CH2)3CH (CH3)2. Compute answers using Wolfram’s breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports, finance, music….